Keywords: Hypertension, Baroreceptor, Piezo channel, Nedd4-2, Baroreflex, Nodose ganglia
Abstract
Baroreflex plays a crucial role in regulation of arterial blood pressure (BP). Recently, Piezo1 and Piezo2, the mechanically-activated (MA) ion channels, have been identified as baroreceptors. However, the underlying molecular mechanism for regulating these baroreceptors in hypertension remains unknown. In this study, we used spontaneous hypertensive rats (SHR) and NG-Nitro-L-Arginine (L-NNA)- and Angiotensin II (Ang II)-induced hypertensive model rats to determine the role and mechanism of Piezo1 and Piezo2 in hypertension. We found that Piezo2 was dominantly expressed in baroreceptor nodose ganglia (NG) neurons and aortic nerve endings in Wistar-Kyoto (WKY) rats. The expression of Piezo2 not Piezo1 was significantly downregulated in these regions in SHR and hypertensive model rats. Electrophysiological results showed that the rapidly adapting mechanically-activated (RA-MA) currents and the responsive neuron numbers were significantly reduced in baroreceptor NG neurons in SHR. In WKY rats, the arterial BP was elevated by knocking down the expression of Piezo2 or inhibiting MA channel activity by GsMTx4 in NG. Knockdown of Piezo2 in NG also attenuated the baroreflex and increased serum norepinephrine (NE) concentration in WKY rats. Co-immunoprecipitation experiment suggested that Piezo2 interacted with Neural precursor cell-expressed developmentally downregulated gene 4 type 2 (Nedd4-2, also known as Nedd4L); Electrophysiological results showed that Nedd4-2 inhibited Piezo2 MA currents in co-expressed HEK 293T cells. Additionally, Nedd4-2 was upregulated in NG baroreceptor neurons in SHR. Collectively, our results demonstrate that Piezo2 not Piezo1 may act as baroreceptor to regulate arterial BP in rats. Nedd4-2-induced downregulation of Piezo2 in baroreceptor NG neurons leads to hypertension in rats. Our findings provide a novel insight into the molecular mechanism for the regulation of baroreceptor Piezo2 and its critical role in the pathogenesis of hypertension.
Introduction
In mammals, the arterial baroreceptors play an essential role in triggering the baroreflex to buffer any deviation of arterial blood pressure (BP). Baroreceptors are stretch-sensitive structures that located at the sensory nerve endings in the wall of aorta arch and carotid sinus. These sensory nerve endings are projected by baroreceptor neurons whose cell bodies are located in the nodose and petrosal ganglia. Baroreceptors sense the stretch of vascular wall caused by BP changing, and then convert the mechanical signals into electrical signals, which propagate to the cardiovascular center. Ultimately, the heart rate (HR), cardiac output, and vascular tone are adjusted via a sympathetic negative feedback to maintain BP stabilization. Baroreflex injury including impairment of baroreceptors leads to overactivity of sympathetic nervous system and results in hypertension. It was reportedly that baroreflex sensitivity impairment is associated with myocardial infarction, heart failure and stroke, and also serve as an indicator for the prognosis of these pathological conditions. Electric field stimulation of baroreceptors (also known as baroreceptor activation therapy) elicits a decrease of BP and reduction of sympathetic activity in patients with drug-resistant hypertension.
The molecular nature of baroreceptor has been studied for decades. Several ion channels such as epithelial sodium channel γ subunit (γENaC), acid sensing ion channel 2 (ASIC2), and TRP channel classical subtype 5 (TRPC5) have been proposed as baroreceptors. However, substantial residual baroreflex is still observed even though these channels are eliminated. None of them could be directly activated by mechanical stimulation in heterologous expression systems. Thus, these channels acting as sensors or downstream signals of mechanotransduction remains controversial. Piezo channels, including Piezo1 and Piezo2, are bona fide mechanically-activated (MA) ion channels that have been identified in 2010 and been extensively studied. Piezo channels are essential for detecting external mechanical stimuli, as well as mechanical forces within tissues. Zeng et al. have identified that Piezo1 and Piezo2 functional together serve as baroreceptors. Double knockout of Piezo1 and Piezo2 results in baroreflex impairment and systolic BP increase in mice.
However, this raises new questions. For example, do baroreceptors of other species need Piezo channels to control BP? What is the role of Piezo baroreceptors in hypertensive animals? The underlying molecular mechanisms for regulating Piezo baroreceptors in hypertension also remains to be determined. In this study, we used hypertensive rats to study the role of Piezo1 and Piezo2 in modulation of arterial BP in baroreceptor neurons and their roles in hypertension. We found that Piezo2 was dominantly expressed in baroreceptor neurons and nerve endings in rats. Piezo2 not Piezo1 acts as baroreceptor to regulate rat arterial BP. Downregulation of Piezo2 by Nedd4-2 in baroreceptor NG neurons could induce hypertension in rat.
Materials and Methods
Animals Male Wistar-Kyoto rats (WKY; total number, 221; 16 weeks of age; bodyweight, 250-300 g) and male spontaneously hypertensive rats (SHR; total number, 31; 16 weeks of age; weight, 250-300 g) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). WKY rats and SHR were housed at room temperature (23 plus or minus 1 degrees Celsius) with a stable humidity (50 plus or minus 5 percent) and free access to food and water on a 12 h/12 h light-dark cycle. Experimental procedures were approved by the Animal Care and Use Committee of Hebei Medical University (Shijiazhuang, China).
Reagents and Antibodies NG-Nitro-L-Arginine (L-NNA; Cat. no. 2149-70-4) was purchased from Yuan Ye Biotechnology Co., Ltd. (Shanghai, China) and Angiotensin II (Ang II; Cat. no. 4474-91-3) was purchased from Med Chem Express (MEC, New Jersey, USA). GsMTx4 (Cat. no. ab141871), nNOS (Cat. no. ab1376) were obtained from Abcam (Ambridge, UK). Antibodies specific to Piezo1 (Cat. no. 15939-1-AP), β-actin (Cat. no. 66009-1-Ig), and Nedd4-2 (Cat. no. 13690-1-AP) were purchased from Protein Tech Group, Inc. (Wu Han, China; dilution, 1:1,000). Antibodies specific to Piezo1 (Cat. no. NBP1-78446) and Piezo2 (Cat. no. NBP1-78624) were purchased from NOVOUS (Basel, Switzerland; dilution, 1:100). Antibody specific to Piezo2 (Cat. no. ARP49683) was purchased from AVIVA (San Diego, USA; dilution, 1:500); and one self-prepared Antibody specific to Piezo2 (dilution, 1:1000). Antibodies specific to Tuj1 (Cat. no. GTX631836) was purchased from GeneTex (Southern California, USA; dilution, 1:500). Scrambled shRNA (r) Lentiviral Particles (Cat. no. Sc-108080), Piezo1 shRNA (r) Lentiviral Particles (Cat. no. Sc-27-342-V) and Piezo2 shRNA (r) Lentiviral Particles (Cat. no. Sc-270372-V) were purchased from Santa Cruz (Santa Cruz, USA). Goat anti-rabbit (Fluorescein 5-isothiocyanate, FITC) (Cat. no. 132373) and Goat anti-mouse (Tetramethylrhodamine, TRITC) (Cat. no. 131512) were purchased from Jackson ImmunoResearch Inc. (Baltimore, USA; dilution, 1:400); SP-DiO (Cat. no. D7778) was purchased from Thermo Fisher Scientific (Waltham, USA). DiI (Cat. no. MB4240) was purchased from Dalian Meilun Biotechnology Co. (Dalian, China).
Hypertensive Animal Model L-NNA-induced hypertensive animal model: In brief, WKY rats (total number, 30), with body weight of 190-210 g, received intraperitoneal (i.p.) injection with L-NNA (7.5 mg/kg/12 hours) for 28 consecutive days. 22 rats had significantly increased BP and were used in the following study; 8 rats were excluded because 5 rats BP fluctuated too much and 3 rats died during the experimental procedure. WKY rats (total number, 30) with the same range of body weight that were intraperitoneally injected with saline (the same volume as L-NNA) for 28 consecutive days were used as control. In control group, 2 rats were excluded because they died during the experimental procedure.
Ang II-induced hypertensive model rats: In brief, WKY rats (total number, 60), with the body weight of 190-210 g, were anesthetized with isoflurane. Osmotic minipumps (Alzet 2002, DURCT, USA) containing either Ang II (400 ng/Kg/min, ANG II group, n equals 30) or the same volume of saline (0.9 percent, control group, n equals 30) were subcutaneously implanted into the animals. benzylpenicillin (19 mg/0.1 mL) was injected into muscular immediately after surgery. Postoperatively, rats were housed individually in plastic cages and supplied with water and food ad libitum. All infusions were performed for 14 days. Arterial BP was measured at the day after 14 days minipump implantation. In Ang II group, 21 rats had significantly increased BP and were used for following study. 9 rats were excluded because 5 rats with damaged osmotic minipumps and 4 rats died during the experimental procedure. In control group, 2 rats were excluded because their osmotic minipumps were damaged.
Measurement of the Hemodynamic Parameters in Rats Carotid catheterization method was used to measure the arterial BP of rats. Briefly, rats were anesthetized with i.p. injection of 2 percent sodium pentobarbital (40 mg/kg). The rats were fixed at supine position and the neck hair was removed. A median incision was made to separate the left carotid artery and vagus nerve. The left carotid artery was separated and two threads were put under the carotid artery for later using. The distal end of the left carotid artery was ligated and the proximal end of the carotid artery was clamped with an arterial clamp. A V shaped incision was made between the distal ligation line and the artery clamp by ophthalmic scissors in the centriolar direction at 45 degrees. The V shaped incision should not be too large, about one-third of the perimeter of the vessel. The polyethylene catheter (PE 50) filled with 2 per mil heparin saline was inserted into the left carotid artery centripetally (about 2 cm) and connected to the pressure transducer (NL108A; Digitimer Ltd.). The arterial BP of rats was recorded and measured (Data acquisition and analysis system, PowerLab).
The heart rates (HR) of rats were measured by electrocardiograph (ECG), which was recorded with three needle electrodes placed subcutaneously on the lower left chest (positive), upper right chest (negative) and left hind paw (ground), respectively and amplified with a differential amplifier (Data acquisition and analysis system, PowerLab).
Immunofluorescence Rats were transcardially perfused with 4 percent PFA under depth of anesthesia (2 percent sodium pentobarbital, 40 mg/kg). Left NG was removed and stored in 4 percent PFA followed by embedding in OCT (SAKURA, Japan). Ten micrometers NG sections were cut using a freezing microtome (Leica, Germany). Sections were washed once with 0.1 mol/L PBS (Beijing Solarbio Science and Technology Co., Ltd.) and punched for 30 min in 37 degrees Celsius with 0.3 percent Triton X-100/PBS buffer and blocked for 1 hours with blocking buffer (10 percent goat serum in 0.1 mol/L PBS). Primary antibodies were diluted in 0.1 percent Triton X-100/PBS buffer before overnight incubation at 4 degrees Celsius. Antibodies of Piezo1 (Cat. no. NBP1-78446), Piezo2 (Cat. no. NBP1-78624), nNOS (Cat. no. ab1376), Nedd4-2 (Cat. no. 13690-1-AP) and Tuj1 (Cat. no. GTX631836) were used in immunofluorescence (IF) assay. The second day, sections received a further 3 times washes in PBS before incubation with secondary antibodies for 2 hours at 37 degrees Celsius. Sections were washed with PBS for 3 times and placed on microscope slides in Vectashield with DAPI (Southern Biotech). Staining was visualized and the IF intensity was measured using a laser scanning confocal microscope (Leica SP5, Leica, Germany).
For aortic arch adventitia, which contains the baroreceptor nerve terminals, was fixed in 4 percent PFA overnight. Then the adventitia was punched for 3h in 1 percent Triton X-100. The procedures for immunostaining were the same as described above.
Real-Time PCR Total RNA was extracted from NG tissues using RNAiso Plus total RNA extraction reagent (Takara Bio, Inc.). cDNA was synthesized using a Prime Script RT reagent Kit with gDNA Eraser (Takara Bio, Inc., Otsu, Japan). Genomic DNA is eliminated by treatment with gDNA Eraser for 2 min at 42 degrees Celsius. The reaction conditions were as follows: 37 degrees Celsius for 15 min, 85 degrees Celsius for 5 sec and 4 degrees Celsius for termination. Subsequently, cDNA was stored at -20 degrees Celsius. qPCR was performed using a SYBR Premix Ex Taq Real-Time PCR Kit (Takara Bio, Inc.). The reaction conditions were one cycle of initial denaturation at 95 degrees Celsius for 3 min, followed by 35 cycles of 95 degrees Celsius for 30 sec, 60 degrees Celsius for 30 sec. The PCR primer sequences were as follows: Piezo1 forward: 3′-CTCCTGTGGAGAACCGTGAT-5′ and reverse: 3′-CTGCGAGTGTTGTAGCCAGT-5′; Piezo2 forward: 3′-TTACATCTGTGCCCTCATCG-5′ and reverse: 3′-CATGGGTACTTCCTCCTGTC-5′; GAPDH forward: 3′-GACATGCCGCCTGGAGAAAC-5′ and reverse: 3′-AGCCCAGGATGCCCTTTAGT-5′. GAPDH was used as an internal control. Expression data were calculated from the cycle threshold (Ct) value, and the 2 to the power of negative delta delta Ct method was used to calculate RNA level.
Co-Immunoprecipitation (Co-IP) and Western Blot Samples were prepared from NG tissues or HEK 293T cells expressing both Piezo2 and Nedd4-2. A certain volume of RIPA (Beyotime Institute of Biotechnology) (containing protease inhibitor) was added to the NG tissues or the collected cells, lysed on ice for 30 min, centrifuged at 12,000 rpm for 30 min, and the supernatant was collected and the concentrations of proteins were detected using a bicinchoninic acid protein kit (Beyotime Institute of Biotechnology).
For Western blot analysis, proteins were extracted from NG, 30 micrograms were used, separated by electrophoresis on 8 percent SDS-polyacrylamide gels, and transferred onto PVDF membranes. Nonspecific binding sites were blocked with 5 percent skim milk in Tris-buffered saline solution with Tween-20 for 2 hours at room temperature. Membranes were then incubated with primary antibodies overnight at 4 degrees Celsius. Antibody for β-actin was used as a housekeeping protein. Antibodies of Piezo1 (cat. no. 15939-1-AP; dilution, 1:500), Piezo2 (cat. no. ARP49683; dilution, 1:500) and β-actin (cat. no. 66009-1-Ig) were used for western blot assay. Second day, the blots were probed with secondary antibodies (IRDye 800CW goat anti-rabbit, Cat. no. 926-32210; IRDye 800CW goat anti-mouse, Cat. no. 926-32211; LI-COR Biosciences), and the blots were visualized using the Odyssey Fc System (LI-COR Biosciences). Densitometry of the protein bands was performed using ImageJ 1.50i software (National Institutes of Health). The experiments were repeated at least three times.
200 micrograms protein samples were used for Co-IP. In this case, the protein was incubated with anti-Piezo2 (self-prepared), anti-Nedd4-2 (Cat. no. 13690-1-AP) and anti-IgG antibodies (each antibody using 3 micrograms) for 16 hours, respectively. Then Protein-G-coated magnetic beads (Sigma, IP50-1KT) were added and the solution was incubated for 12 hours. The protein was denatured with SDS buffer containing 50 mM Tris-HCl, 2 percent SDS, 100 mM DTT, 10 percent glycerol and 0.01 percent bromophenol blue for 10 minutes at 95 degrees Celsius and then separated by SDS-PAGE. Anti-Piezo2 (self-prepared) antibody and anti-Nedd4-2 (13690-1-AP) antibody were detected. The experiments were repeated at least three times.
Retrograde Labelling of Aortic Baroreceptor NG Neurons The procedures were similar to previously described. Briefly, rats were anesthetized with 2 percent sodium pentobarbital (40 mg/kg). Nerves in the left cervical area were exposed. 3-4 mm of the aortic depressor nerve was carefully detached from surrounding tissues. The fluorescent dye 1,1′-dioleyl- 3,3,3,’3′-tetramethylindo-carbocyanine methane sulfonate (DiI) crystals were applied around the left aortic depressor nerve with a parafilm underneath, and the area was wrapped by a rapid-curing gel (Kwik-Sil, World Precision Instruments). The incision was sutured afterward. The animals were recovered for 10 days to allow dye to diffuse retrogradely along the aortic depressor nerve to the soma located in NG.
Single-cell RT-PCR Single-cell RT-PCR was performed as previously described. Retrogradely labeled NG neurons were aspirated (under a microscope) into a patch pipette using a conventional patch-clamp setup with negatively pressurised pipette holder. The electrode tip was then quickly broken into a 0.2-mL PCR tube containing 0.7 microliters of oligo-dT (50 mM), 1 microliter of dNTP mixture (10 mM), 0.5 microliter of MgCl2 (25 mM), 0.7 microliter of RNaseOUT (40 U/mL), and 1.4 microliters of DEPC-treated water; the mixture was heated to 65 degrees Celsius for 5 minutes and then placed on ice for 1 minute. Single-strand cDNA was synthesized from the cellular mRNA by adding 0.5 microliter of RT buffer, 1.5 microliter of MgCl2 (25 mM), 1 microliter of DTT (1M), 0.5 microliter of RNaseOUT (40U/mL), and 1 microliter of Super Script III RT (200 U/mL) and then incubating the mixture at 55 degrees Celsius for 50 minutes followed by 85 degrees Celsius for 5 minutes. Synthesis of single-cell cDNA was performed using a C1000 Touch thermal cycler-CFX96Real-time PCR (BIO-RAD, Hercules, CA). First strand synthesis was executed at 95 degrees Celsius (1 minute) followed by 40 cycles (95 degrees Celsius for 50 seconds, 60 degrees Celsius for 50 seconds, and 72 degrees Celsius for 55 seconds) and a final 10 minutes elongation at 72 degrees Celsius by adding the specific outer primer pairs into each PCR tube. Then, 2.5 microliters of the product of the first PCR was used in the second amplification round by using specific inner primers (final volume 25 microliters). The second amplification round consisted of heating the samples to 95 degrees Celsius (1 minute) followed by 40 cycles (95 degrees Celsius for 50 seconds, 60 degrees Celsius for 50 seconds, and 72 degrees Celsius for 55 seconds) and 10-minute elongation at 72 degrees Celsius. The products of the second PCR were analysed in 2 percent agarose gels and stained with ethidium bromide. SuperScript III First-Strand Synthesis System Kit and GoTaq Green Master Mix were obtained from Takara-Clontech (Invitrogen, Carlsbad, CA) and Promega (Madison, WI), respectively.
Cells Culture and Transfection Primary cultures of NG neurons were prepared from rats (about 250 g) as previous studies described. In brief, rats were deep anesthetized and then the ganglia were rapidly removed and placed into the D-Hank’s solution (Gibco). Ganglia samples were digested at 37 degrees Celsius with collagenase (2 mg/mL, Worthington) for 30 min. They were subsequently suspended in 10 mL DMEM (Gibco) plus 10 percent bovine calf serum (Gibco) to stop digestion. Ganglia were then dissociated into a suspension of individual cells and plated onto 13 mm glass coverslips pre-coated with 100 micrograms per mL poly-D-lysine (BD Biosciences). Cells were incubated at 37 degrees Celsius with a 5 percent CO2 and 95 percent O2. Retrogradely labelled NG neurons by DiI dye were used for patch clamp recording within 24 hours.
HEK 293T cells were plated onto 13 mm glass coverslips pre-coated with poly-D-lysine (100 micrograms per mL). Transfections were performed with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. In addition, EGFP was co-transfected to identify the transfected cells. The Piezo2 plasmid was purchased from Addgene (plasmid number 81073). The Nedd4-2 (also known as Nedd4L) plasmid was purchased from Youbao Biological Technology Co., Ltd. (NM-001114386).
Electrophysiology Whole-cell mode patch clamp recordings were performed at a room temperature of 22-24 degrees Celsius as previous study described. Coverslips with cultured NG neurons were placed in a 0.5 ml microchamber, mounted on a stage of an Olympus IX70 inverted microscope (Olympus Co, Japan) and continuously perfused at 2 ml/min with bath solution. The bath solution contained (in mM) 145 NaCl, 5 KCl, 2 MgCl2, 2 CaCl2, 10 HEPES and 10 glucose with an osmolarity 320 mOsm. The pH was adjusted to 7.35 with NaOH. The internal recording solution contained (in mM) 70 Cs2SO4, 5 KCl, 2.4 MgCl2, 0.5 CaCl2, 5 EGTA, 10 HEPES, 5 Na2ATP, 0.33 GTP-Tris salt, pH was adjusted to 7.35 with CsOH and osmolarity was adjusted with sucrose to 320 mOsm. The recording electrodes were pulled from thick wall borosilicate glass capillaries using a Flaming P-97 puller (Sutter Instrument Co. Novato, CA). Resistances of electrodes were 3-5 mega ohms after filling recording internal solutions. The protocol used to record MA currents in baroreceptor NG neurons was as follows: the cells that were retrogradely labelled by DiI were selected to record MA currents. The cells were held at -60 mV and cell membranes were displaced by a heat-polished glass probe. The probe, with a approximately 4 micrometers diameter tip, was positioned at an angle of 45 degrees to the dish surface. Its movement was controlled by a Piezo-electric device (Physik Instrumente, Ltd.). Cells were stimulated with a series of mechanical stimuli in 1 micrometer increments to elicit MA currents. The moving velocity of the probe was set at 0.5 micrometers per millisecond. The probe and the recorded cells were visualized as live images on a monitor throughout the recording. The live images were captured continuously through a CCD camera that was connected to the 40× microscope objective. To record the Piezo2 MA currents, Piezo2 with or without Nedd4-2 were expressed in HEK 293T cells. The bath solution, internal recording solution and the recording protocol were as same as demonstrated above. Signals were recorded with an Axopatch 700B amplifier, filtered at 2 KHz and sampled at 5 kHz using pCLAMP 10.7 (Axon Instruments; Molecular Devices, LLC).
Injection of shRNA Lentivirus or GsMTx4 or Yoda1 in NG In brief, WKY rats were anesthetized with i.p. injection of 2 percent sodium pentobarbital (40 mg/kg). A midline incision was made at cervical area of rats. Both nodose ganglions were exposed. The microinjector (Hamilton Co.) contained 2 microliters solution of shRNA lentiviruses particles (approximately 1×10 to the power of 6 copies of Control shRNA, Piezo1 shRNA or Piezo2 shRNA) was focally inserted into both nodose ganglions to a depth of 500 micrometers. The shRNA lentiviral particles were injected slowly into NG, and the needle was removed 5 minutes after the injection was complete. The incision was closed with sutures. Intramuscular injection of benzylpenicillin (19 mg/0.1 mL) was given immediately after surgery. Postoperatively, rats were housed individually in plastic cages and supplied with water and food ad libitum. After infection for 15 days, the common carotid artery cannulation method was used to measure SBP, DBP, MAP and PP.
Arterial BP was measured from the right common carotid artery cannulation of the rats. The left NG was exposed. After the BP was stabilized, 1 microliter GsMTx4 (NS as control) or Yoda1 (5 per mil DMSO as control) (100 micromole per liter, 0.25 microliters per min) was injected into the NG, and changes in blood pressure were observed after GsMTx4 or Yoda1 injection.
Evaluation of Baroreflex Rats infected with lentivirus particles (Piezo1-, Piezo2- and scrambled shRNA) in NG after anesthetic. 2 weeks later, the rats were fixated at supine position, the left carotid artery was exposed and arterial BP was recorded as above; The HR of rats was measured by ECG. When the BP was stable, PE was slowly administered through the left femoral vein (rate: 200 microliters per min; infusion dose: 50 micrograms per kg). Then, the BP increased sharply and the HR slowed down reflexively. The BP and HR were continuously recorded until the BP recovered. The baroreceptor sensitivity was evaluated by calculating the changes of HR and systolic blood pressure after intravenous injection of PE.
Enzyme Linked Immunosorbent Assay (ELISA) The peripheral venous blood (about 3 ml) of rats was collected. Samples were incubated at room temperature for 2 hours and then centrifuged at 2000× g for 20 mins. The supernatant was then collected to detect the level of serum norepinephrine (NE) with the ELISA kit (Cloud-Clone corp. Cat. no. HEA907Ge) based on the kit instructions. Briefly, the standards, reagents and samples were prepared before the experiment. 50 microliters samples (including standard and sample to be tested) and 50 microliters detection reagent A (prepared before using) were added to each well, then incubated at 37°C for 1 hour. The wells were washed three times using washing solution; then 100 microliters detection reagent B was added to each well and incubated at 37°C for 30 minutes. After this, the wells were washed 5 times using washing solution; then 90 microliters substrate solution was added to each well and incubated at 37°C for 20 minutes. The reaction was terminated by adding 50 microliters stop solution to each well and the absorbance was immediately read at 450 nm.
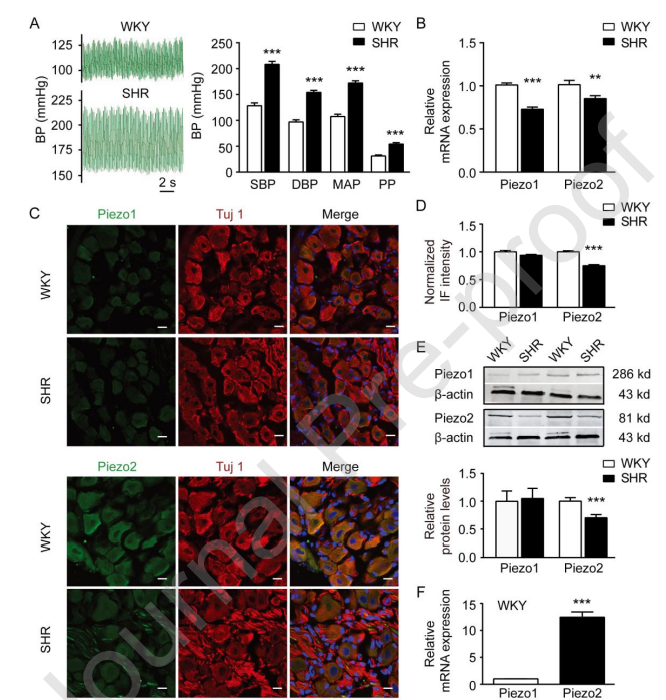
Figure 1 demonstrates that the expression of Piezo2 is significantly downregulated in nodose ganglion (NG) neurons of spontaneously hypertensive rats (SHR) compared to normotensive Wistar Kyoto (WKY) rats.Panel (A) on the left displays representative blood pressure (BP) tracings from both WKY rats (upper trace) and SHR (lower trace), showing elevated BP in the SHR group. The right side of panel (A) summarizes key blood pressure parameters — systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), and pulse pressure (PP) — all measured using the carotid artery intubation method. These values confirm that SHRs (n = 10) exhibit significantly higher blood pressure than WKY rats (n = 9).Panel (B) presents relative mRNA expression levels of the mechanosensitive ion channels Piezo1 and Piezo2 in the NG. While Piezo1 levels are comparable between groups, Piezo2 mRNA expression is notably reduced in SHR (n = 11) compared to WKY rats (n = 12), suggesting selective downregulation.Panel (C) shows representative immunofluorescence (IF) images of Piezo1 (top row) and Piezo2 (bottom row) proteins in NG neurons. Tuj1 staining serves as a neuronal marker. In these images, Piezo2 expression appears visibly weaker in SHR neurons relative to WKY, while Piezo1 shows little difference.Panel (D) quantifies the normalized IF signal intensities for Piezo1 and Piezo2. Quantitative analysis supports the imaging data: Piezo2 intensity is significantly lower in SHR (n = 124) compared to WKY (n = 86), whereas Piezo1 intensity is not significantly altered (n = 155 in SHR, n = 106 in WKY).Panel (E) provides Western blot data, including both representative blots and summary quantification, reinforcing the reduction of Piezo2 protein in the SHR group (n = 8 per group), with no significant change in Piezo1 protein levels (n = 6 per group).
Statistical Analysis Data were presented as the Mean plus or minus SEM and analyzed using GraphPad Prism 8. Statistical significance was evaluated using either a Student’s t-test or one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc test for multiple groups or two-way ANOVA followed by Bonferroni’s multiple comparisons test. The chi squared test was used to assess the differences in the proportion of Piezo2-positive baroreceptor NG neurons between WKY rats and SHR. The chi squared test was also used to assess the differences in the proportion of RA-MA and non-responsive (NR) neurons in cultured NG neurons transfected with scrambled shRNA and Piezo2 shRNA. P less than 0.05 was considered to indicate a statistically significant difference.
Results
Piezo2 Was Dominantly Expressed in NG Neurons in WKY Rats and Downregulated in Hypertensive Rats Recent studies have shown that both Piezo1 and Piezo2 MA channels are obviously expressed in the mouse NG neurons. In the present study, SHR and L-NNA- or Ang II-induced hypertensive model rats were used to address the different expression of Piezo1 and Piezo2 in NG between normotensive WKY rats and hypertensive rats. The carotid artery intubation method was used to record the BP of WKY rats and SHR. The SBP, DBP, MAP and PP were all significantly higher in SHR than that of WKY rats (Figure 1A). qPCR assay showed that the mRNA of both Piezo1 and Piezo2 in the NG of SHR were significantly lower than that of WKY rats by 27.8 percent (P less than 0.001) and 15.2 percent (P less than 0.01), respectively (Figure 1B). Immunostaining assays were also performed (Figure 1C). In the NG neurons of WKY rats, the immunofluorescence (IF) of Piezo2 was obviously displayed, however, the IF of Piezo1 was hardly detected (Figure 1C). Moreover, the IF intensity of Piezo2 in the NG neurons of SHR was significantly reduced by 25 percent (P less than 0.001), while, the IF intensity of Piezo1 had no significant alterations (Figure 1C and D). Both qPCR and immunostaining assays indicated that Piezo2 expressed at higher levels in the NG of WKY rats and significantly downregulated in the NG of SHR (Figure 1B-D). Consistent with these results, western blot assay clearly showed that Piezo2 but not Piezo1 dominantly expressed in the NG of WKY rats, and its expression was significantly reduced by 32.2 percent (P less than 0.001) in the NG of SHR (Figure 1E). Indeed, qPCR assay showed that Piezo2 expression was about 12.5-fold higher than that of Piezo1 (P less than 0.001) at the mRNA level in the NG of WKY rats (Figure 1F). These results indicate that Piezo2 dominantly expresses in NG neurons of normotensive WKY rats and significantly downregulated in NG neurons of SHR.
We then used drug-induced hypertensive model rats to address whether Piezo2 expression is also downregulated in their NG neurons. As shown in Figure 2A and 2B, L-NNA- and Ang II-induced hypertensive animal models were performed. These two types of hypertensive model rats were characterized with higher SBP, DBP, MAP and PP (Figure 2A and B). Similar to SHR, qPCR, immunostaining and Western blot assays congruously showed that the expression of Piezo2 in the NG neurons of both L-NNA- and Ang II-induced hypertensive rats was significantly downregulated while Piezo1 kept no changes (Figure 2C-K). The Piezo2 mRNA in the NG of L-NNA- and Ang II-induced hypertensive model rats were decreased by 24.0 percent (P less than 0.001) and 25.6 percent (P less than 0.05), respectively (Figure 2C and D). The IF intensity of Piezo2 in the NG neurons of L-NNA- and Ang II-induced hypertensive rats were reduced by 32.7 percent (P less than 0.001) and 15.8 percent (P less than 0.001), respectively (Figure 2G and H). In addition, the Piezo2 protein were decreased by 12.3 percent (P less than 0.05) and 30.0 percent (P less than 0.05), respectively in the NG of L-NNA- and Ang II-induced hypertensive model rats (Figure 2I-K). Taken together, these results demonstrate that Piezo2 not Piezo1 dominantly expresses in NG neurons of normal BP rats. Piezo2 is significantly downregulated in hypertensive rats, which strongly indicates that Piezo2 expression in NG neurons may play an essential role in the development of hypertension.
The Expression and Activity of Piezo2 MA Channels Were Downregulated in Baroreceptor NG Neurons in Hypertensive Rats NG neurons includes different types of sensory neurons innervated the lungs, gastrointestinal tract, heart and aortic arch. The NG neurons innervating aortic arch are named baroreceptor neurons, and its nerve endings express the baroreceptors. In addition, NG baroreceptor neurons specifically expressed neuronal isoform of nitric oxide synthase (nNOS), therefore nNOS-positive NG neurons could represent baroreceptor neurons. To test the alteration of Piezo1 and Piezo2 in NG baroreceptor neurons in hypertensive rats, immunostaining assays were performed in nNOS positive NG neurons. In WKY rats, Piezo1 and Piezo2 were almost expressed in all nNOS-positive NG neurons (Figure 3A and B), and 34.8 percent of Piezo1- and 30.0 percent of Piezo2-positive NG neurons were overlaped with nNOS (Figure 3A-C). Neither nNOS/Piezo1- nor nNOS/Piezo2-positive populations of neurons showed significant difference between WKY rats and SHR (Figure 3C, P greater than 0.05). In SHR, the IF intensity of Piezo2 in nNOS-positive NG neurons was significantly reduced by 30.2 percent (P less than 0.001), while no significant changes were observed for Piezo1 (P greater than 0.05) (Figure 3D).
Piezo1 and Piezo2 were identified as bona fide mammalian mechanically-activated (MA) ion channels. We then performed patch clamp recordings to address the MA currents in aortic baroreceptor NG neurons and to compare the difference in MA currents between WKY rats and SHR. Based on their inactivation kinetics (also known as decay time constant, tau), the MA currents were classified into three types, including rapidly adapting (RA, tau less than 10 ms), intermediately adapting (IA, 10 ms less than tau less than 30 ms) and slowly adapting (SA, tau greater than 30 ms). Piezo1 and Piezo2 were sufficient to mediate IA- and RA-MA currents, respectively. In this experiment, retrogradely labelled aortic baroreceptor NG neurons by DiI dye from the aortic depressor nerve were selected for evoking MA currents with mechanical stimulation (Figure 4A and B). In WKY rats, 46.8 percent (29 out of 62) of baroreceptor NG neurons showed RA-MA currents. Only small amount of baroreceptor NG neurons (9.7 percent and 8.1 percent, respectively) displayed IA- and SA-MA currents (Figure 4C and D). The proportion of RA-MA type baroreceptor NG neurons was significantly reduced to 20.5 percent in SHR (Figure 4C and D, P less than 0.05). While the proportion of non-responsive (NR) baroreceptor NG neurons was significantly increased from 35.5 percent in WKY rats to 68.2 percent in SHR (Figure 4D; P less than 0.05). There were no significantly differences in the percentages of baroreceptor NG neurons with IA- and SA-MA currents between WKY rats and SHR (6.9 percent for IA-MA, P greater than 0.05 and 4.6 percent for SA-MA, P greater than 0.05) (Figure 4D). More importantly, the RA-MA current densities of baroreceptor NG neurons were significantly reduced in SHR (Figure 4C and E). There was approximately 5-fold reduction on the RA-MA current density at the membrane displacement stimulation of 13 micrometers (Figure 4E, P less than 0.05). Furthermore, single-cell PCR was performed with the retrogradely labelled baroreceptor NG neurons. Consistent with the electrophysiological analysis, the proportion of Piezo2-positive neurons was markedly reduced from 89.4 percent in WKY rats to 66.7 percent in SHR (P less than 0.01). Taken together, these results indicate that the expression and function of Piezo2/RA-MA channel are downregulated in aortic baroreceptor NG neurons in SHR. Moreover, the Piezo1/IA-MA current densities of baroreceptor NG neurons have no significant change between WKY and SHR. While the SA-MA current densities of baroreceptor NG neurons were also significantly reduced in SHR, which was consistent with the findings of a previous study.
To further determine the RA-MA currents were mediated by Piezo2 channels in NG neurons, we recorded the whole-cell MA currents from cultured NG neurons which were transfected with scrambled shRNA and Piezo2 shRNA (n equals 33 neurons for scrambled shRNA and n equals 48 neurons for Piezo2 shRNA), respectively. After knocking down the expression of Piezo2 by shRNA, the proportion and current amplitude of RA-MA currents were significantly reduced (proportion in Piezo2 shRNA group, 18.8 percent, compared with that of scrambled shRNA group, 39.4 percent, P less than 0.05). While, the proportions of IA- and SA-MA currents kept no significant changes.
The Expression of Piezo2 Was Downregulated in Aortic Baroreceptor Nerve Endings We then tested the expression of Piezo1 and Piezo2 and their alterations in the aortic baroreceptor nerve endings in normotensive WKY rats and SHR by immunostaining assay. The aortic baroreceptor nerve endings were specifically labelled by 3,3′-Dioctadecyl-5,5′-Di(4-Sulfophenyl) Oxacarbocyanine, Sodium Salt (SP-DiO). In WKY rats, Piezo2 was obviously identified in aortic baroreceptor nerve endings, whereas Piezo1 was hardly detected (Figure 5A-C), which was consistent with the characteristics of Piezo1 and Piezo2 expression in baroreceptor NG neurons. Moreover, Piezo2 expression was significantly downregulated in aortic baroreceptor nerve endings of SHR (70.7 plus or minus 5.1 A.U.) compared with WKY rats (140.9 plus or minus 15.9 A.U., P less than 0.001), while, Piezo1 expression did not show any alterations (Figure 5A-C). Taken together, these results strongly suggest that Piezo2 not Piezo1 may act as baroreceptor in the baroreceptive reflex to modulate arterial BP.
Knocking Down or Blocking Piezo2 MA Channels in NG Resulted in Hypertension Above results suggested that the Piezo2 could be the modulator of BP. Inhibition of the function of Piezo2 theoretically could upregulate the BP through impaired baroreflex. To test this hypothesis, carotid artery intubation method was used to measure the arterial BP in rats. Firstly, the Piezos inhibitor GsMTx4 (100 micromole per liter, 2 microliters) was slowly injected into the left NG of WKY rats and the arterial BP was significantly elevated (Figure 6A and B). The alteration of SBP (delta SBP), delta DBP, delta MAP and delta PP were significantly increased in GsMTx4 group (21.9 plus or minus 6.5, 14.6 plus or minus 5.3, 19.1 plus or minus 5.0 and 7.2 plus or minus 3.3 mmHg, respectively) compared to that of control group (-3.4 plus or minus 2.0, P less than 0.01; -1.3 plus or minus 1.6, P less than 0.05; -2.3 plus or minus 0.9, P less than 0.01 and -2.0 plus or minus 1.2 mmHg, P less than 0.05; respectively). (Figure 6A and B). While Yoda1 (the specific Piezo1 agonist) showed no effects on the arterial BP when it was slowly injected into NG in WKY rats. These results indicate that targeting Piezo2 not Piezo1 may modulate arterial BP through affecting baroreflex.
To determine the exact role of Piezo1 and Piezo2 in regulating arterial BP in long term, shRNA lentiviral particles were directly injected into NG to knock down the expression of Piezos. WKY rats was randomly divided into three groups (Scrambled, Piezo1 and Piezo2 shRNA) after evaluation of BP with tail-cuff method. Scrambled shRNA lentiviral particles were used as control. After infection for 15 days, the expression of Piezo1 and Piezo2 in NG was significantly reduced by shRNA interference. Importantly, the BP (SBP, DBP and MAP) and serum norepinephrine (NE) concentrations in Piezo2 shRNA group rats rather than Piezo1 shRNA group rats were significantly elevated (Figure 6C-E). These results further indicate that Piezo2 not Piezo1 may act as baroreceptor and its downregulation results in hypertension.
To evaluate whether baroreceptor sensitivity was affected by knocking down Piezos in NG, we tested the function of baroreflex in response to PE among the above three groups of rats. Intravenous infusion of PE at a rate of 200 microliters per min (50 micrograms per kg) produced a transient increase in SBP and a consequent decrease in heart rate (HR) through baroreflex (Figure 6F). In Piezo2 shRNA group rats, the SBP elevation induced by PE (94.5 plus or minus 1.9 mmHg) was significantly higher than control (77.1 plus or minus 1.7 mmHg, P less than 0.05) (Figure 6F and G). The PE-induced reduction in HR (287 plus or minus 12 bpm for scrambled shRNA group versus 134 plus or minus 6 bpm for Piezo2 shRNA group, P less than 0.01) and baroreflex sensitivity (delta HR divided by delta SBP, 3.8 plus or minus 0.2 bpm per mmHg for Scrambled shRNA group versus 1.4 plus or minus 0.1 bpm per mmHg for Piezo2 shRNA group, P less than 0.01) was obviously attenuated in Piezo2 shRNA group rats (Figure 6F and G). Whereas, Piezo1 shRNA group rats did not show any significant alterations in the SBP (76.9 plus or minus 1.8 mmHg, P greater than 0.05), HR (247 plus or minus 8 bpm, P greater than 0.05) and baroreflex sensitivity (3.3 plus or minus 0.1 bpm per mmHg, P greater than 0.05) (Figure 6F and G). Taken together, these results strongly indicate that Piezo2 rather than Piezo1 act as baroreceptor and its down-regulation significantly impaired the baroreflex and induced hypertension in rats.
Nedd4-2 Was Involved in the Downregulation of Piezo2 in NG in Hypertensive Rats Next, we explored the mechanism underlying the downregulation of Piezo2 in baroreceptor NG neurons in hypertensive rats. Piezo2 possesses a conserved PY-motif, PPSY (at the site of 1828-1831, 1796-1799 and 1898-1901 in human, rat and mouse Piezo2, respectively), located in the intracellular domain (Figure 7A). PPSY is well known to interact with the WW domains of E3 ubiquitin ligase Nedd4-2. Once binding to Nedd4-2, the PY-motif were preferentially orchestrated their internalization and subsequent degradation or recycling by Nedd4-2. Numerous studies have demonstrated that ion channels possessing PY-motifs (such as KCNQ, ENaC and voltage-gated sodium channel (Nav) (Figure 7A) were downregulated by Nedd4-2. Therefore, we hypothesis Piezo2 could be downregulated by Nedd4-2.
To test the hypothesis, we first tested the possible interaction between Piezo2 and Nedd4-2 in native NG and in HEK 293T cells co-expressed Piezo2 and Nedd4-2. Co-IP analysis revealed that both Piezo2 and Nedd4-2 bands were detected in IP-Nedd4-2 and IP-Piezo2 experiments either in HEK 293T cells co-expressed Piezo2 and Nedd4-2 (Figure 7B) or in rat NG (Figure 7C). Nedd4-2 and Piezo2 obviously pulled down each other both in HEK 293T cells co-expressed Piezo2 and Nedd4-2 (Figure 7B) and in rat NG (Figure 7C). In addition, immunostaining assays found that the IF intensity of Nedd4-2 was significantly increased in the nNOS-positive baroreceptor NG neurons of SHR (109.8 plus or minus 5.8 A.U., P less than 0.001), compared with WKY rats (79.8 plus or minus 4.1 A.U.) (Figure 7D). These results indicated that Nedd4-2 interacted with Piezo2 in baroreceptor NG neurons and negatively regulated Piezo2 expression in NG neurons of SHR.
To further determine Nedd4-2 negatively regulated Piezo2, whole-cell patch clamp experiments were performed in HEK 293T cells, which were expressed Piezo2 plus Nedd4-2 or alone. Indeed, Piezo2 MA current densities were significantly reduced in Piezo2 co-expression with Nedd4-2 group by 64.8 percent compared to Piezo2 group under 10-micrometer mechanical stimulation (Figure 8A and B). Moreover, the threshold (P less than 0.01) and latency (P less than 0.05) for evoking Piezo2 MA currents were significantly increased in Piezo2 co-expression with Nedd4-2 group (Figure 8C). There was no significant difference in the current decay time between these two groups (P greater than 0.05) (Figure 8C). Taken together, these results suggested that Nedd4-2 negatively regulated Piezo2 MA channel activities.
Discussion The major findings of this study are as following: 1) Piezo2 rather than Piezo1 is dominantly expressed in baroreceptor NG neurons in rats; 2) downregulation of Piezo2 expression in baroreceptor NG neurons induces hypertension in rats; 3) Nedd4-2, at least partially, is involved in the downregulation of Piezo2 in baroreceptor NG neurons, by which may result in hypertension in rats.
In mammals, baroreceptors initiate the baroreflex to buffer any deviation of arterial BP through the negative feedback control of sympathetic and parasympathetic activities. Baroreceptors are mechanotransduction device that located in baroreceptor nerve terminals. It can sense the stretch stimulation from the vascular wall induced by phasic pulsatile changes as well as acute fluctuation of arterial BP and convert these mechanical signals into electrical signals. Previous studies have reported that γENaC, ASIC2, and TRPC5 are likely to serve as baroreceptors. However, mechanically activated Piezo channels (both Piezo1 and Piezo2) are recently identified as bona fide baroreceptors to regulate arterial BP. Double knockout of Piezo1 and Piezo2 results in labile hypertension and increases BP variability. Thus, the molecular nature of baroreceptor has been re-evaluated, though some criteria of baroreflex function are not fully satisfied by Piezo1/Piezo2 yet. In the present study, we have further studied the role of Piezo1 and Piezo2 in baroreceptor neurons and nerve terminals in regulation of BP using normotensive and hypertensive rats. Interestingly, our results displayed that Piezo2 is dominantly expressed in NG neurons and baroreceptor nerve terminals. And the majority of baroreceptor NG neurons in rats have rapidly adapting type of mechanically-activated (RA-MA) currents (Figure 4) which are mediated by Piezo2. To our best knowledge, this is the first time to describe RA-MA currents in baroreceptor neurons. Furthermore, Piezo2 expression as well as RA-MA current densities have been significantly reduced in hypertensive rats. In normotensive rats, the arterial BP was significantly elevated either by inhibiting Piezo2 MA channel activity or by knocking down Piezo2 expression in baroreceptor NG neurons. In contrast, the expression of Piezo1 in baroreceptor neurons and nerve terminals is hardly detected in normotensive rats and does not show any difference from that of hypertensive rats. Moreover, knockdown of Piezo1 in baroreceptor NG neurons does not induce any changes in arterial BP. Overall, our study indicates that Piezo2 not Piezo1 may act as baroreceptor to regulate arterial BP in rats. Our study demonstrates the species specificity of Piezo1 and Piezo2 baroreceptors between rats and mice. However, it is hard to know the precise role of Piezo1 and Piezo2 in baroreflex in human due to the limitations. In mouse, Piezo1 and Piezo2 express at similar levels in baroreceptor neurons, but disruption of Piezo1 or Piezo2 alone in baroreceptor neurons has no effect on BP. One plausible explanation is that Piezo1 and Piezo2 may compensate each other under the single gene knockout condition to maintain normal force-sensing function. Increasing evidence demonstrates this view is logical. For example, TRPV4 compensates for the deficiency of TRPV3 in cochlear hair cells to maintain normal hearing in mice. In hypertensive rats, Piezo1 expression may be too low to compensate the downregulation of Piezo2 there by impairs baroreflex.
It has been suggested over time that baroreceptor mechanism only contributes to short-term (within seconds and minutes) BP regulation. This point is originally proposed in the 1970s by Cowley, that complete arterial bar oreceptor denervation does not affect long-term (within days) BP regulation. However, accumulating evidence suggests that baroreflex can participate in long-term regulation of BP. For example, chronic electric stimulation of baroreceptors continuously lowers the BP in hypertensive dogs, rabbits, and patients. It has also been shown that changes in the sensitivity of the baroreflex control loop may contribute to the development of hypertension in animal models. Impairment of baroreceptor function leads to BP elevation. However, intact baroreceptors can reset to the elevated BP level in hypertensive rats. Resetting of baroreceptors implies a change of threshold and operating point of baroreceptors, which contributes to maintaining the chronic hypertension. In our study, we found that Piezo2 channel activities and expression in baroreceptor neurons were significantly downregulated in hypertensive rats. These results suggest that the downregulation of Piezo2 may contribute to the resetting of baroreceptors and the maintenance of high BP in hypertensive rats. Our findings provide new evidence supporting the role of baroreflex in long-term regulation of arterial BP.
The molecular mechanisms underlying the downregulation of Piezo2 in hypertensive rats remain largely unknown. In this study, we found that Nedd4-2 was involved in the downregulation of Piezo2 in baroreceptor NG neurons in hypertensive rats. Nedd4-2 is an E3 ubiquitin ligase that belongs to the Nedd4 family. Nedd4-2 regulates the degradation and trafficking of several ion channels and receptors through ubiquitination. Studies have shown that Nedd4-2 can regulate ENaC, voltage-gated sodium channels, KCNQ channels, and ACE2. The interaction between Nedd4-2 and its substrates depends on the PY motif (PPXY or PPXpY) in the substrates and the WW domains in Nedd4-2. Sequence analysis revealed that Piezo2 possesses a conserved PY motif (PPSY) in its intracellular domain. Our co-immunoprecipitation experiments confirmed that Piezo2 interacts with Nedd4-2 both in native NG tissues and in HEK 293T cells co-expressing Piezo2 and Nedd4-2. More importantly, we found that Nedd4-2 expression was upregulated in baroreceptor NG neurons in SHR. Electrophysiological experiments showed that co-expression of Nedd4-2 with Piezo2 significantly reduced Piezo2 MA current densities and increased the threshold and latency for evoking Piezo2 MA currents. These results suggest that Nedd4-2 negatively regulates Piezo2 channel activities through direct interaction. The upregulation of Nedd4-2 in baroreceptor neurons may be one of the important mechanisms responsible for the downregulation of Piezo2 in hypertensive rats.
The mechanism by which Nedd4-2 is upregulated in hypertensive rats remains to be determined. Previous studies have shown that Nedd4-2 expression can be regulated by various factors, including aldosterone, angiotensin II, serum and glucocorticoid-regulated kinase, and oxidative stress. In the present study, we used three different hypertensive rat models including SHR, L-NNA-induced hypertensive rats, and Ang II-induced hypertensive rats. We found that Piezo2 expression was downregulated in all three hypertensive models, suggesting that the downregulation of Piezo2 may be a common mechanism in different types of hypertension. However, whether the upregulation of Nedd4-2 is mediated by different pathways in different hypertensive models needs further investigation.
It is worth noting that in addition to Nedd4-2, other mechanisms may also contribute to the downregulation of Piezo2 in hypertensive rats. For example, inflammatory factors, oxidative stress, and changes in calcium homeostasis have been shown to affect the expression and function of ion channels. Whether these factors are also involved in the regulation of Piezo2 in hypertension warrants further study. Moreover, post-translational modifications such as phosphorylation, glycosylation, and lipidation may also regulate Piezo2 channel activities. Future studies are needed to fully elucidate the mechanisms underlying the regulation of Piezo2 in hypertension.
Our study has some limitations. First, although we demonstrated that Piezo2 downregulation in baroreceptor neurons leads to hypertension, the exact cellular and molecular mechanisms by which Piezo2 senses mechanical stimulation from the vascular wall and converts it into electrical signals remain to be fully elucidated. Second, while we showed that Nedd4-2 interacts with Piezo2 and negatively regulates its activity, the detailed mechanisms by which Nedd4-2 promotes Piezo2 degradation or internalization need further investigation. Third, whether targeting Nedd4-2 or Piezo2 could be a potential therapeutic strategy for hypertension needs to be tested in future studies.
Conclusion
In conclusion, our study demonstrates that Piezo2 channel is dominantly expressed in baroreceptor NG neurons in rats and plays an essential role in regulating arterial BP. Downregulation of Piezo2 in baroreceptor neurons impairs baroreflex and leads to hypertension. Nedd4-2 interacts with Piezo2 and negatively regulates its expression and activity. The upregulation of Nedd4-2 in baroreceptor neurons may be responsible for the downregulation of Piezo2 in hypertensive rats. These findings provide new insights into the molecular mechanisms underlying baroreceptor dysfunction in hypertension and suggest that targeting Piezo2 or Nedd4-2 could be potential therapeutic strategies for hypertension treatment.
