Geniposide Alleviates Hepatocyte Oxidative Damage: Role of the miR-27b-3p/Nrf2 Axis and ML385 Intervention
ABSTRACT: Geniposide (GEN), a main compound extracted from Gardenia jasminoides fruit, has various biological activities including anti-inflammation, cellular damage alleviation, neuroprotection, and others. However, the effect of GEN on oxidative stress in hepatic cells is yet to be investigated. Our study uncovered that GEN eliminated excess intracellular free radicals by activating the Nrf2/ARE signaling pathway in H2O2-treated hepatocytes, while the protective effect was blocked by ML385 (an inhibitor of Nrf2). Moreover, H2O2 led to upregulation of miR-27b-3p in L02 cells, which was restrained by GEN. Overexpression of miR-27b-3p greatly weakened the antioxidant capacity of GEN in hepatocytes via directly targeting the Nrf2 gene. Our findings indicated that GEN treatment recovered H2O2-induced oxidative stress via targeting miR-27b-3p and thereby enhanced the antioxidant capacity by stimulating nuclear translocation and accumulation of Nrf2. These findings suggest that inhibition of miR-27b-3p to activate the Nrf2/ARE pathway by GEN is a potential alternative for hepatic oxidative damage alleviation.
INTRODUCTION
Liver injury has become an important medical problem, as it further causes liver fibrosis, cirrhosis, and even liver cancer that seriously affects human health. Oxidative stress response refers to the cellular process in which cells exert some antioxidant molecular expressions during oxidative stress to protect themselves. As the center of human metabolism, the liver involves many redox processes accompanied by a large amount of reactive oxygen species ( ROS ) production such as superoxide anion, hydroxyl free radical, and hydrogen peroxide. Under pathological stress, excess ROS are produced and cannot be cleared in time, which in turn causes detrimental effects, leading to disorders of liver metabolism. In short, oxidative stress is crucial to the pathogenesis of several hepatic diseases such as alcoholic hepatitis, nonalcoholic steatohepatitis (NASH), viral hepatitis, etc.
The Keap1-Nrf2-ARE signaling pathway is the central regulator of cellular antioxidant response, which is regarded as a potential target for metabolic syndrome therapy. Under normal circumstances, Nrf2 is located in the cytoplasm and interacts with Kelch-Like ECH-Associated Protein 1 (Keap1), which is then rapidly degraded following a ubiquitin proteasome pathway. After getting attack signals from reactive oxygen species or nucleophiles, Nrf2 dissociates from Keap1 and then translocates into the nucleus and binds with other bZIP proteins to form heterodimers.
The antioxidant responsive element (ARE) in the promoter region of antioxidant genes binds with the targeted genes of post-transcriptional activation to produce glutathione-S-transferase (GST) and heme oxygenase-1 (HO-1) to protect cells from oxidative stress. A large number of studies have found that the Keap1-Nrf2-ARE pathway is an indispensable part of defense mechanism against various environmental and endogenous stress, as well as has a wide range of anti-inflammatory, antistress, antiapoptotic, and neuroprotective function.
MicroRNA ( miRNA ) is a class of single-stranded noncoding small RNAs approximately 19−25 nucleotides in length that regulates protein expression by binding imperfectly complementary to the 3′-untranslated region (3′-UTR) of their target genes and promoting their degradation and/or translational repression. In recent years, a large number of endogenous miRNAs have been identified, and each miRNA can regulate multiple target genes, thereby regulating different biological processes, such as proliferation, differentiation, and metabolism.
Thus, miRNA dysregulation often leads to cellular dysfunction and disease development. Since the discovery of miR-27b-3p , its aberrant expression has been observed in cellular dysfunctions and diseases, including oxidative stress, breast cancer, and lung cancer. In addition, it was reported that Nrf2 is a direct target gene of miR-27b-3p. It is attractive to hypothesize that there is crosstalk between oxidative stress and miR-27b-3p.
Gardenia is widely used as a food coloring and health food in Eastern countries. It can also be used as a natural yellow dye and has a variety of biological activities. Gardenia jasminoides has complex chemical components, and geniposide (GEN) is a type of iridoid glucoside extracted from the gardenia plant. It has been reported that GEN possesses beneficial effects like antiallergy, anti-inflammation, and hepatoprotection.
Moreover, GEN is readily soluble in water, and a sufficient concentration can directly pass through the blood−brain barrier to exert a neuroprotective effect. Interestingly, no detailed research has shown the favorable effectiveness of GEN on oxidative stress in hepatocytes. It is important to understand the underlying molecular mechanism of GEN on hepatic oxidative damage elimination. Thus, the protective effects of GEN against an in vitroH2O2-induced hepatic injury model (L02 cells) were the main focus of this work. Then the regulation of GEN on the Nrf2/ARE antioxidant signaling pathway and expressions of miR-27b-3p were investigated.
MATERIALS AND METHODS
Chemicals. Geniposide ( GEN ), dimethyl sulfoxide ( DMSO ), 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide ( MTT ), and naphthalene-2,3-dicarboxaldehyde ( NDA ) were purchased from Aladdin (Shanghai, China). Dihydroethidium ( DHE ) and 2′,7′-dichlorofluorescin diacetate ( DCFH-DA ) were purchased from Sigma-Aldrich (St. Louis, MO, USA). 5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanineiodide ( JC-1 ), qPCR SYBR Green Master Mix (No Rox), dual luciferase reporter gene assay kit, and liposomal transfection reagent were purchased from Yeason (Shanghai, China), while the Nrf2 inhibitor was obtained from Aladdin (ML385, Shanghai, China). Mito-Tracker Green ( MTG ), pARE-luc-CP reporter vector, pRL-TK reporter vector, and bicinchoninic acid ( BCA ) protein assay kit were obtained from Beyotime Institute of Biotechnology, Ltd. (Shanghai, China). A tissue/cell miRNA extraction kit was purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China). Other reagents were of analytical grade.
Cell Culture. The Cell Bank of Type Culture Collection of Chinese Academy of Sciences provided the human liver cell line HL-7702 (L02). Cells were cultured in RPMI 1640 media containing 10% fetal bovine serum, 100 units/mL of penicillin and streptomycin, and 37 °C with 5%CO2.
Cell Transfection.MiR-27b-3pmimics (5′-UUCACAGUGGCUAAGUUCUGC-3′) and the corresponding negative control were chemically synthesized by Tsingke Biotechnology Co., Ltd. (Hangzhou, China). Cells were seeded into 6-well cell culture plates at a density of3 times 10^5cells/well. The transfection of miRNA mimics was performed using liposomal transfection reagent according to the standard protocols provided by the manufacturer. Then the cells were incubated for 48 h at 37 °C. Next, L02 cells were treated with 100muM GEN solutions for 24 h and then exposed toH2O2(0.4 mM) for a further 6 h. Then the cell viability and levels of ROS were detected. Real-time PCR was used to measure the expression level ofmiR-27b-3pThe expression levels ofNrf2,p-Nrf2,GCLC, andHO-1were also detected by Western blot.
Cell Viability Assay. The MTT assay was used to determine cell viability. L02 cells were seeded into 96-well cell culture plates. After the treatments, the cells underwent two phosphate-buffered saline ( PBS ) washes before being incubated with 0.5 mg/mL MTT for 4 h. Finally, DMSO was added to each well to dissolve the formazan precipitate. A microplate reader (Tecan, Switzerland) was used to measure the absorbance at 490 nm.
Determination of ROS and Superoxide Anion Radicals. Cellular ROS was detected by the nonfluorescent DCFH-DA . In a 24-well cell culture plate, L02 cells were cultured at a density of4 times 10^4cells per well. Following treatments, the cells were washed twice with PBS , treated withDCFH-DA(10muM) for 30 min, then washed twice with PBS , and immediately evaluated under a fluorescent microscope. A DHE probe was used to analyze cellular generation of superoxide anion radicals. In brief, L02 cells received identical treatment to that described for ROS measurement. The mean fluorescence intensity was used to express the results, which were calculated using Image-Pro Plus 6.0 (Media Cybernetics, Inc., Singapore).
Determination of Glutathione ( GSH ). The previously described GSH method was applied using a GSH -sensitive probe NDA to measure the intracellular GSH levels. L02 cells were treated with different concentrations of GEN (0, 25, 50, 100muM). Cells were then dyed with NDA and observed under a fluorescence microscope after that. By comparing the changes of fluorescence intensity of the control, GSH levels of samples were determined.
Determination of Mitochondrial Membrane Potential ( MMP ) and Mitochondrial Mass. JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanineiodide) and MTG fluorescent probes were used to determine the intracellular MMP and mitochondrial mass. Briefly, L02 cells with6 times 10^4cells/well density were cultured in 24-well cell culture plates for 24 h. Then different concentrations of GEN (0, 25, 50, 100muM) were used to treat cells. Then cells were incubated with 0.4 mMH2O2for 6 hours. After Gen andH2O2treatments, the cells were washed with PBS and incubated withJC-1(6mug/mL) or MTG (1muM) for 30 min and washed twice with PBS , and the cells were evaluated right away under a fluorescence microscope.
Dual Luciferase Reporter Assay. L02 cells were seeded in 24-well culture plates and cotransfected with 0.5mug of pARE-luc-CP reporter vector and 0.05mug of pRL-TK reporter vector for 24 h followed by treatment of 100muM GEN for 24 h. Then L02 cells were incubated with 0.4 mMH2O2for 6 h. After treatment, cells were lysed, and the dual luciferase reporter assay system kit (Yeasen, China) was used to measure luciferase activity. Luciferase activity was normalized to the renilla luciferase activity.
Treatment with an Nrf2 Inhibitor. An Nrf2 inhibitor ( ML385 ) was dissolved in DMSO at the concentration of 10 mM and diluted to 2.5muM with RPMI 1640 media before use. The cells were treated with 2.5muM ML385 for 0.5 h, followed by exposure to 100muM GEN for a further 24 h. Then 0.4 mMH2O2was used to treat the cells for 6 h. Subsequently, cell viability, ROS , and related protein expression were determined.
Western Blot Analysis. The total protein of cells was extracted with RIPA lysate, and the nuclear protein of the cells was extracted with the nuclear and cytoplasmic protein extraction kit (Beyotime Institute of Biotechnology, Shanghai, China). The concentration of protein was quantified by a BCA protein assay kit (Solarbio Science & Technology, Beijing, China). Briefly, electrophoresis on sodium dodecyl sulfate−polyacrylamide gels was used to separate 20mug of cell protein samples, and the separated proteins were then transferred to polyvinylidene fluoride membranes.
After blocking the membranes with 5% bovine serum albumin in PBS containing Tween 20 ( PBST ) for 1 h, the membranes were incubated overnight at 4 °C with the following primary antibodies:Keap 1(Abcam, ab227828), Nrf2 (Abcam, ab62 352),p-Nrf2(Abcam,ab76026),GCLC(Abcam,ab207777),HO-1(Abcam,ab52947), GAPDH (Abcam,ab181602), andLamin B(Abcam,ab133741). The membranes were incubated with a secondary antibody that was horseradish peroxidase ( HRP ) -conjugated for an hour at room temperature after three PBST washes .
Bioinformatics Prediction and Real-Time qPCR ( RT-qPCR ). The probable miRNAs that target the 3′UTR ofNrf2were predicted using the tools TargetScan, miRanda, miRDB, and DIANA. To increase fidelity, the putative miRNAs were thought to be indicated by the intersection of the prediction results obtained from these four tools. MicroRNA was extracted from cells with tissue/cell miRNA extraction kit. Then qPCR was carried out with the miRNA 1st strand cDNA synthesis kit (by stem-loop) and qPCR SYBR Green Master Mix following the manufacturer’s protocols.
Statistical Analysis. The meanpmstandard deviation ( SD ) from three separate studies was used to express the results. One-way variance ( ANOVA ) analysis was performed to determine the statistical significance (p < 0.05(using SPSS 22.0)
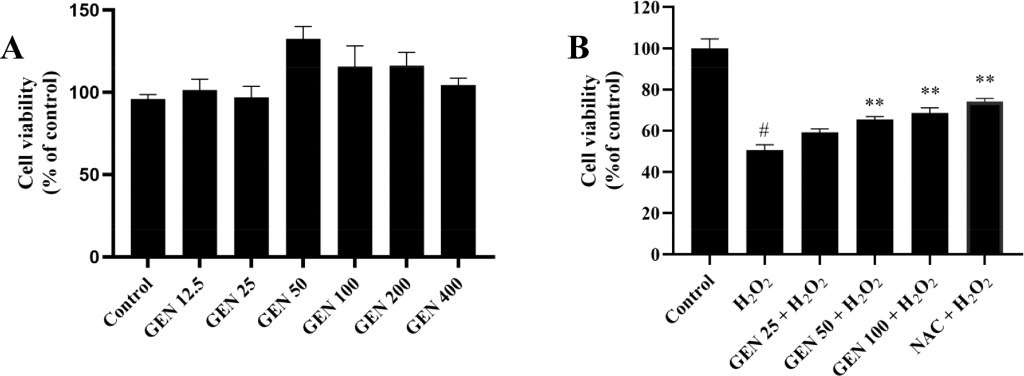
Figure 1. Effect of GEN onH2O2-induced toxicity in L02 cells. (A) Effect of GEN (12.5, 25, 50, 100, 200, and 400muM) on cell viability. (B) Protective effect of GEN (25, 50, and 100muM) onH2O2-induced cytotoxicity. L02 cells were treated with GEN solutions for 24 h and then exposed toH2O2(0.4 mM) for a further 6 h. The cell viability was measured by the MTT assay. Control group was considered as 100%; P < 0.05 represents the significant difference compared with the control group;P < 0.05represents the significant difference compared with theH2O2-treated group;P < 0.01represents highly significant difference compared with theH2O2-treated group. GEN , geniposide.
Figure 2. Effect of GEN onH2O2-induced ROS accumulation in L02 cells. L02 cells were treated with GEN (25, 50, and 100muM) for 24 h and exposed to 0.4 mMH2O2for 6 h. After that, the cells were incubated with a fluorescent probe for 30 min and evaluated immediately using a fluorescence microscope. (A,B) Effect of GEN onH2O2-induced ROS generation measured by 10muM DCFH-DA fluorescent probe. The results were expressed as the mean DCF fluorescence intensity (n = 6(C,D) Effect of GEN onH2O2-induced superoxide anion radical generation measured by 10muM DHE fluorescent probe. The results were expressed as the mean DHE fluorescence intensity (n = 6). Control group was considered as 100%; #P < 0.05 represents the significant difference compared with the control group;P < 0.05represents the significant difference compared with theH2O2-treated group;P < 0.01represents the highly significant difference compared with theH2O2-treated group. GEN , geniposide.
RESULTS AND DISCUSSION
Effects of Geniposide onH2O2-Induced Hepatic Oxidative Stress. First, we observed that the L02 cell viability did not significantly decrease after exposure to GEN with various doses for 30 h compared with untreated cells, showing that GEN had no cytotoxicity in the concentration range from 12.5 to 400muM (Figure 1A). Establishing an in vitro hepatic cell damage model has been considered as oxidative damage is a common pathophysiological basis of liver injury. A suitable concentration ofH2O2(0.4 mM) that led to approximately 50% cell death was chosen for the following research. To evaluate the effect of GEN onH2O2-induced hepatic oxidative stress, L02 cells were treated with GEN for 24 h and then exposed toH2O2for a further 6 h, followed by the MTT assay. Figure 1B demonstrates that pretreatment with GEN increased cell viability significantly compared withH2O2treatment alone. The cell viability was separately elevated to59.2 pm 2.2%(25muM GEN – treated group),65.4 pm 2.4%(50muM GEN -treated group), and68.7 pm 2.4%(100muM GEN – treated group) from50.6 pm 1.9%(H2O2-treated group), unveiling the potential protective effect of GEN againstH2O2-induced oxidative stress. It has been reported that GEN prevented human melanocytes fromH2O2-induced oxidative damage, which is consistent with our result, and the potential protective mechanism was next to be explored.
Geniposide SuppressedH2O2-Induced ROS Accumulation. According to the meanDCFfluorescence intensity, ROS accumulation was found to be significantly increased inH2O2-treated L02 cells (312.0 pm 4.2%of control) (Figure 2A,B). This effect was significantly inhibited by the pretreatment with 25, 50, and 100muM GEN , and the mean fluorescence intensities of GEN were232.2 pm 10.2,178.2 pm 5.8, and166.0 pm 13.8%of the control, respectively. Furthermore, superoxide anion radicals, which can result in oxidative damage, were found in the cells using the DHE probe. The mean DHE fluorescence intensity was decreased to485.2 pm 2.7%of the control in the presence of 100muM GEN compared to solelyH2O2treatment640.2 pm 17.9%of control). All of these findings indicated that GEN might preventH2O2-induced ROS formation in L02 cells.
Geniposide AmelioratesH2O2-Induced GSH Depletion. In order to explore the effect of GEN on the level of GSH in L02 cells under oxidative stress, the NDA fluorescent probe was used to detect the level of GSH in cells . with 0.4 mMH2O2, the intracellular GSH level was significantly reduced, and the NDA fluorescence intensity was only28.3 pm 1.5%(the fluorescence intensity of the blank control group was set to100.0%). Pretreatment with 2 mM NAC (a precursor of GSH ) effectively increased the level of intracellular GSH compared to that with theH2O2-treated group, which indicates that NAC can effectively slow down theH2O2-induced GSH depletion. Similarly, GEN pretreatment also increased the intracellular GSH levels. The NDA fluorescence intensities of 50 and 100muM GEN pretreatment groups were95.6 pm 6.7and96.6 pm 8.1%, respectively.
Figure 3. Effect of GEN on GSH levels and mitochondrial membrane potential in L02 cells. L02 cells were treated with GEN (25, 50, and 100muM) for 24 h and exposed to 0.4 mMH2O2for 6 h. After that, the cells were incubated with a fluorescent probe for 30 min and evaluated immediately using a fluorescence microscope. (A,B) Effect of GEN onH2O2-induced depletion of GSH measured by a 50mug/mL NDA fluorescent probe. The results were expressed as the mean NDA fluorescence intensity (n = 6(C) CellsJC-1red and green fluorescent image (times 200); (D) Quantitative data of panel (C). Control group was considered as 100%; P < 0.05 represents the significant difference compared with the control group;P < 0.05represents the significant difference compared with theH2O2-treated group;P < 0.01represents the highly significant difference compared with theH2O2-treated group. GEN , geniposide.
Geniposide AmelioratesH2O2-Induced Mitochondrial Membrane Potential Decreased. In this study, the JC-1 fluorescent probe was used to detect the effect of GEN on the MMP in L02 cells under cytotoxic injury. When the MMP is high,JC-1forms aggregates in the mitochondrial matrix and emits red fluorescence, whereas green fluorescence is produced whenJC-1is a monomer under low MMP . The red/green fluorescence intensity decreased sharply to39.84%of control afterH2O2treatment, while GEN pretreatment partly recovered this decline (Figure 3C,D). The green fluorescence intensity of Mito-Tracker Green is proportional to the number of cellular mitochondria.
The results of this study showed that oxidative stress induced byH2O2and the protection process by GEN did not involve the number of mitochondrial changes (FigureS1). A major source of oxidants and a target for detrimental effects are mitochondria. The loss of mitochondrial function, as seen by reduced mitochondrial oxidative phosphorylation, ineffective electron transport, and further increased oxidant flux, results from mitochondria continuous oxidative stress. According to Lv et al., GEN protects against mitochondrial dysfunction inAPP/PS1mice by reducing oxidative damage to the mitochondria and increasing MMP . According to our study, GEN exhibited beneficial effects via inhibiting cellular ROS generation and improving MMP levels in the L02 cells, which suggests that GEN could act as an antioxidant that can target mitochondria to suppress hepatic oxidative damage.
Geniposide Activated the Nrf2/ARE Signaling Pathway. When cells suffer from oxidative damage, they produce a series of protective proteins to scavenge the excessive ROS , while ARE is the core regulatory element. ARE is a specific DNA prompter binding sequence, which was being inhibited not only byH2O2but also by compounds that can provoke oxidative stress in the body or metabolize active electrophilic products. This is because ROS and endogenous active molecules are continuously produced during normal aerobic metabolism, and ARE plays an important role in maintaining the intracellular redox balance. Through nuclear translocation ofNrf2, the Nrf2/ARE signaling pathway is activated. Thus, we further determined the nuclearNrf2protein level and ARE promoter activity in L02 cells to verify the activation of Nrf2/ARE signaling pathway by the GEN .
As expected, cells pretreatment with 25, 50, and 100muM GEN elevated the nuclearNrf2protein level to1.61 pm 0.33,1.91 pm 0.40, and2.39 pm 0.30times the control, respectively (Figure 4D,E). The levels of nuclearNrf2protein were remarkably higher in the GEN -treated than they were in theH2O2-treated groups. Our results suggest that GEN might be a potentNrf2activator, so next the ARE promoter activity was analyzed. Cell pretreatment with 25, 50, and 100muM GEN enhanced the ARE promoter activity compared toH2O2-treated group (Figure 4F). Additionally,Nrf2’s release fromKeap1upon phosphorylation atSer-40confirms its nuclear translocation and theARE-mediated cellular antioxidant response. We wondered whether the phosphorylation ofNrf2or inhibition ofKeap1is involved in the activation of Nrf2/ARE signaling pathway of GEN . Therefore, the levels ofKeap1and phosphorylatedNrf2(p-Nrf2) atSer-40in L02 cells were measured by Western blot. As shown in Figure 4B,C, pretreatment with GEN significantly increased the expression of cellularp-Nrf2protein. Thep-Nrf2protein levels in 25, 50, and 100muM GEN -treated groups were0.89 pm 0.09,1.08 pm 0.20, and1.48 pm 0.02times the control, respectively. On the contrary, theKeap1protein level in pretreated cells with GEN was not significantly different compared to theH2O2-treated group (FigureS2). Therefore, we speculate that GEN exerts its antioxidant effect mainly by promoting phosphorylation and nuclear translocation ofNrf2rather than by affecting the expression ofKeap1.
Nrf2as a transcription factor positively regulated a large number of cytoprotective downstream genes includingHO-1and glutamate−cysteine ligase (Gcl).HO-1is a rate-limiting enzyme in heme catabolism, which converts heme to biliverdin,CO, and ferrous iron. Biliverdin is then converted to bilirubin by biliverdin reductase. Both biliverdin and bilirubin can inhibit lipid and protein peroxidation by scavenging ROS .GclcandGclm, as the modifier and a catalytic subunit ofGcl, perform the first and rate-limiting step during GSH synthesis by catalyzing the formation ofgamma-glutamylcysteine from glutamine and cysteine. Moreover,Nrf2plays an important role in the liver, as the liver is the mainly responsible for antioxidants and detoxification. To further investigate the effect of GEN on the Nrf2/ARE signaling pathway, we detected the expressions ofNrf2downstream genes and proteins, includingGclcandHO-1in L02 cells. As shown in Figure 4A−C, GEN (100muM) treatment significantly increased the cellular gene and protein expressions ofGclcandHO-1, confirming the activation of Nrf2/ARE signaling pathway by GEN pretreatment.
Figure 4. Effect of GEN on Nrf2/ARE signaling pathway in L02 cells. (A) Effects of GEN (25, 50, and 100muM) onNrf2,GCLC, andHO-1gene expressions. GAPDH was used as an internal control for gene expression. (B,C) Effect of GEN (25, 50, and 100muM) on totalNrf2,p-Nrf2,GCLC, andHO-1protein expression. GAPDH was used as an internal control for proteins. (D,E) Effect of GEN (25, 50, and 100muM) on nuclearNrf2expression.Lamin Bwas used as an internal control for nuclear proteins. (F) Effect of GEN on ARE promoter activity in L02 cells. #P < 0.05 represents the significant difference compared with the control group;P < 0.05represents the significant difference compared with theH2O2-treated group;P < 0.01represents the highly significant difference compared with theH2O2-treated group. GEN , geniposide.
Nrf2 Inhibitor Inhibits the Protective Effect of Geniposide onH2O2-Induced Oxidative Stress. Here, we investigated whether GEN preventedH2O2-induced cytotoxicity by regulating the Nrf2/ARE signaling pathway using anNrf2inhibitor ( ML385 ). Using the MTT assay, we evaluated howML385affected cell viability and selected the optimal concentration (2.5muM) with no cytotoxicity (Figure 5A). Our findings indicated thatML385treatment eliminated GEN ’s ability to protect L02 cells fromH2O2-induced oxidative damage (Figure 5B). The protective effect of GEN onH2O2-induced ROS accumulation was abolished byML385(Figure 5C,D). Furthermore,ML385treatment revised the protective effect of GEN on total and phosphorylationNrf2, its downstream gene expression (Figure 5E−G), as well as ARE promoter activity (Figure 5H). These results collectively suggested that GEN could promote theNrf2phosphorylation, nuclear translocation, and ARE promoter activity to activate the Nrf2/ARE signaling pathway.
As an excellent plant resource that has the concomitant function of both medicine and foodstuff, Gentiopicroside (GEN) is commonly used to treat hypertension, contusions, lacerations, erysipelas, and icteric hepatitis.
Commonly extracted from plants in the Gentiana genus, GEN is a secoiridoid glycoside known for its broad pharmacological profile:
- Anti-inflammatory: GEN can inhibit the inflammatory response induced by lipopolysaccharide (LPS) in Raw264.7 macrophages by modulating pathways such as NF-κB.
- Antioxidant: It suppresses the elevation of reactive oxygen species (ROS) under UV-B irradiation in human dermal fibroblast cell lines, protecting skin cells from oxidative stress and photoaging.
- Hepatoprotective: It is particularly valued in treating liver disorders, including icteric hepatitis (jaundice), by reducing fibrosis and protecting hepatocytes.
These therapeutic properties make GEN a significant focus of research at centers like the National Center for Complementary and Integrative Health (NIH), where the efficacy of natural antioxidants and anti-inflammatory compounds is evaluated for modern clinical applications.
Recent studies have shown that GEN also has certain effects such as against cardiovascular diseases, antioxidant, scavenging free radicals, inhibiting platelet aggregation, regulating blood lipids, and preventing atherosclerosis. Previous research shows that GEN can be absorbed and converted into genipin in the bowel, implying that genipin may be the active form of GEN in blood. Wang et al. suggest that genipin could induce theNrf2/HO-1expression to inhibit the inflammatory markers inLPS-treatedBV2glial cells. Jiang et al. also reported that genipin inhibited the expression of inflammatory parameters through theHO-1signal pathway to prevent the occurrence of atherosclerosis. Our study demonstrated that GEN alleviatedH2O2-induced oxidative injury in L02 cells through the activation of Nrf2/ARE signaling pathway, indicating thatNrf2is the key target for GEN . Considering practical absorption and utilization, more in vitro simulation and in vivo studies are still needed to investigate activities of GEN .
Figure 5. Effect ofNrf2inhibitor (ML385) against cytoprotective effects of GEN . (A) Effects ofML385(1, 2.5, 5, 10, 15, 20, and 30muM) on cell viability. (B) Effect ofML385against cytoprotective effects of GEN in L02 cells. (C,D) Effect ofML385onH2O2-induced ROS generation measured by a 10muM DCFH-DA fluorescent probe. The results were expressed as the meanDCFfluorescence intensity (n = 6(E)ML385inhibited gene expressions of GEN -treated Nrf2/ARE signaling pathway in L02 cells. GAPDH was used as an internal control for gene expression. (F,G)ML385inhibited protein expression in GEN -treated Nrf2/ARE signaling pathway in L02 cells. GAPDH was used as an internal control for proteins. (H)ML385inhibited ARE promoter activity in GEN -treated L02 cells. #P < 0.05 represents the significant difference compared with the control group;P < 0.05represents the significant difference compared with theH2O2-treated group. GEN , geniposide.
Prediction of Putative MiRNAs Using Bioinformatics that Target the 3′UTR ofNrf2. TargetScan, miRanda, miRDB, and DIANA prediction tools identified 9, 128, 81, and 20 miRNAs that target the 3′UTR ofNrf2, respectively. Combining the results of prediction analysis with these four tools led to the identification of 6 miRNAs (miR-27b-3p,miR-27a-3p,miR-142-5p,miR-340-5p,miR-153-3p, andmiR-144-3p) (Figure 6A). The expression ofmiR-27b-3pwas increased inH2O2-treatedL02cells (7.385 pm 1.011of control) according to the relative expression levels (Figure 6B). This effect was strikingly inhibited by the pretreatment with 50 and 100muM GEN , and the relative expression levels of GEN were4.732 pm 0.776and1.605 pm 0.628of the control, respectively. The other five candidate miRNAs did not respond to GEN , althoughmiR-27a-3p,miR-142-5p, andmiR-340-5pThey also increased!H2O2-treatedL02cells (FigureS3It was reported thatmiR-27b-3pplayed a role in a variety of illnesses by influencing cell proliferation, invasion, and metastasis. For example,miRNA-27b-3pinhibits chondrocyte apoptosis in rheumatoid arthritis by targetingHIPK240. These results collectively manifested that GEN could inhibit the expression ofmiR-27b-3pinL02cells.
MiR-27b-3p Overexpression Suppresses the Nrf2/ARE Signaling Pathway. In the present study, we observed that the expression ofmiR-27b-3pwas significantly increased aftermiR-27b-3pmimics transfection for 48 h in comparison to negative control (Figure 6C). A suitable concentration ofmiR-27b-3pmimics (50 nM) that could increase the expression ofmiR-27b-3pto91.83 pm 8.469in comparison to control was chosen for the following research. As shown in Figure 6D−G, the gene expression and protein levels ofNrf2were decreased after transfection withmiR-27b-3pmimics compared with the negative control, and the ARE promoter activity was decreased, thereby inhibiting the expression ofNrf2downstream genes and proteins. These results collectively manifested thatmiR-27b-3poverexpression could suppress the Nrf2/ARE signaling pathway inL02cells.
MiR-27b-3p Overexpression Inhibits the Protective Effect of Geniposide onH2O2-Induced Oxidative Stress. The underlying mechanisms of biological effects of GEN have been extensively studied. In addition, several molecules and signaling pathways are regulated by GEN . For example, by modulating theNrf2/AMPK/mTORsignaling pathway, GEN could alleviateNAFLD. In various human illnesses, such as diabetic cognitive impairment, acute liver injury, and others, theTLR4/MyD88/NF-Bpathway is regulated by GEN . In addition, several studies have reported the correlation between GEN treatment and miRNA.MiR-124,miR-155, andmiR-224were all regulated by GEN . GEN can improve rheumatoid arthritis by inhibitingItgbeta/Ras-Erk1/2signaling pathways by regulatingmiRNA-124a, and it has been reported that GEN could protect against depression by suppressing inflammation through themiRNA-155-5p/SOCS1axis.
Figure 6. Bioinformatics prediction of the putative miRNAs that target the 3′UTR ofNrf2. (A) MicroRNAs targeting the 3′UTR ofNrf2were predicted using TargetScan, miRanda, miRDB, and DIANA tools; six miRNAs targetingNrf2were predicted by intersection analysis. (B) Effects of GEN (50 and 100muM) on the expression levels ofmiR-27b-3p(C) Expression levels ofmiR-27b-3paftermiR-27b-3pmimics transfection for 48 h. (D−F) Effect ofmiR-27b-3poverexpression on Nrf2/ARE signaling pathway. GAPDH was used as an internal control. (G) Effect ofmiR-27b-3poverexpression on ARE promoter activity. #P < 0.05 represents the significant difference compared with the control group;P < 0.05represents the significant difference compared with theH2O2-treated group;P < 0.01represents the highly significant difference compared with theH2O2-treated group. GEN , geniposide.
We next assessed the function of miR-27b-3p/Nrf2 in the progression of GEN-regulated oxidative stress in L02 cells. Our results indicated that miR-27b-3p overexpression abolished the protective effect of GEN against H2O2-induced oxidative stress in L02 cells (Figure 7A). DCFH-DA staining demonstrated that miR-27b-3p overexpression abolished the protective effect of GEN on H2O2-induced ROS accumulation (Figure 7B,C). Furthermore, we found that GEN-pretreated increased gene and protein expression of Nrf2, GCLC, and HO-1, which were downregulated by miR-27b-3p overexpression (Figure 7D−F). The ARE promoter activity was downregulated after miR-27b-3p overexpression (Figure 7G). Together, these findings demonstrated that GEN-improved oxidative stress is mainly dependent on Nrf2 negatively regulated by miR-27b-3p.
Figure 7. MiR-27b-3p overexpression inhibits the protective effect of geniposide on H2O2-induced oxidative stress. (A) Effect of miR-27b-3p overexpression against cytoprotective effects of GEN in L02 cells. (B,C) Effect of miR-27b-3p overexpression on H2O2-induced ROS generation measured by a 10 muM DCFH-DA fluorescent probe. The results were expressed as the mean DCF fluorescence intensity (n = 6). (D−F) MiR-27b-3p overexpression inhibited gene and protein expressions in GEN-treated Nrf2/ARE signaling pathway in L02 cells. GAPDH was used as an internal control. (G) MiR-27b-3p overexpression inhibited ARE promoter activity in GEN-treated L02 cells. #P < 0.05 represents the significant difference compared with the NC group; P < 0.05 represents the significant difference compared with the H2O2-treated group. NC, negative control; GEN, geniposide.
It has been reported that GEN could ameliorate liver fibrosis through reducing the oxidative stress in C57BL/6 male mice. After 4 weeks of treatment, GEN significantly reduced the liver index, hepatic MDA level, and liver function markers (AST and ALT), as well as significantly increased the GSH-Px activity compared with liver fibrosis model mice. Zhang et al. also reported that GEN could alleviate the alcohol-induced liver injury through reducing oxidative stress in male konmin mice. Their GEN treatment suppressed the MDA level and increased the GSH/GSSG ratio. Chen et al. uncovered that GEN showed comparative beneficial effects against CCl4-induced liver damage via improving antioxidant defense. These in vivo studies ensured that GEN could be a promising candidate for exerting a protective effect against oxidative damage.
To sum up, this work for the first time evaluated GEN as a powerful antioxidant that can alleviate hydrogen peroxide-induced hepatic oxidative damage using L02 cells. GEN treatment suppressed the excessive free radicals and recovered the imbalance of mitochondrial membrane potential. Moreover, GEN pretreatment up-regulated the HO-1 and GCLC expressions at mRNA and protein levels, which are mediated via Nrf2/ARE. Inhibition of protective effects of GEN on H2O2-induced L02 cells by the Nrf2 inhibitor (ML385) further confirmed the effectiveness of GEN treatment. Furthermore, H2O2 induced the upregulation of miR-27b-3p to inhibit Nrf2, leading to excess ROS production. GEN decreased miR-27b-3p expression to activate the Nrf2/ARE pathway, protecting against oxidative damage. Therefore, our study supports a novel notion that GEN could be a food-derived functional component for hepatoprotection by the inhibition of miR-27b-3p. Further research is needed to confirm the in vivo antioxidant capacity of GEN through regulating the miR-27b-3p/Nrf2 axis.
